Brine mining has the potential to add a significant revenue stream to SWRO – and possibly be a more profitable business than the production of fresh water itself – while simultaneously reducing the amount of brine discharged into the sea and increasing SWRO plants’ freshwater output.
That’s why Saudi Arabia’s SWCC recently announced a major initiative to start brine mining in connection with its Jubail 2 desalination plant, which in addition to fresh water is expected to produce two million tons of sodium chloride and 3,600 tons of bromine annually. Indeed, other SWRO operators from Spain to Indonesia are beginning to mine their brines. Other valuable chemicals extractable from seawater, albeit in smaller quantities, include magnesium and potassium.
How is brine mining different than traditional mining methods?
Brine mining is an alternative to traditional and much more widespread mining techniques such as surface and underground excavation.
Like all mining, its purpose is to extract valuable minerals from the earth. However, instead of searching for minerals in dry-rock ores, brine miners look for these as solids dissolved in brines, or concentrated solutions of salts in water.
Such salts include sodium chloride (NaCl) – the stuff we sprinkle on our food – but in chemical terms refer to any inorganic or organic compound with ionic bonds. Of course, we don’t want most of these salts anywhere near our dinner tables, but we do rely on the many chemicals derived from them in all kinds of other ways.
Brine mining is a profitable way to obtain valuable minerals and compounds
Strictly speaking, brine mining began millennia ago when people discovered they could use the sun’s heat to evaporate seawater, our planet’s most abundant brine, and crystalize salt for use in preparing and preserving food. The same artisanal processes are still in use now in many countries.
Today, however, most brine mining is carried out at a much greater scale by large industrial operations that use specialized equipment to concentrate, extract, and process a range of minerals and compounds from brines that might be located far from the sea. In Chile and Argentina, for example, the brine mining process for lithium begins with drilling wells to access the underground brine deposits, followed by brine pumping to the surface for further processing. The brine is then treated to concentrate and finally isolate lithium hydroxide.
Advances in technology, environmental restrictions on surface and underground mining, the depletion of easily obtainable dry-rock ores, and rising commodity prices have made it increasingly feasible and financially attractive to extract some minerals from brines.
While all commodity prices fluctuate, demand generally continues to rise along with population growth and industrialization. This includes sodium chloride and many other compounds found in brines. In some cases, notably for metals critical to electricity generation, storage, and distribution, price developments are even more volatile. For example, prices for lithium hydroxide, essential for batteries, increased more than five-fold between mid-2020 and end-2022.
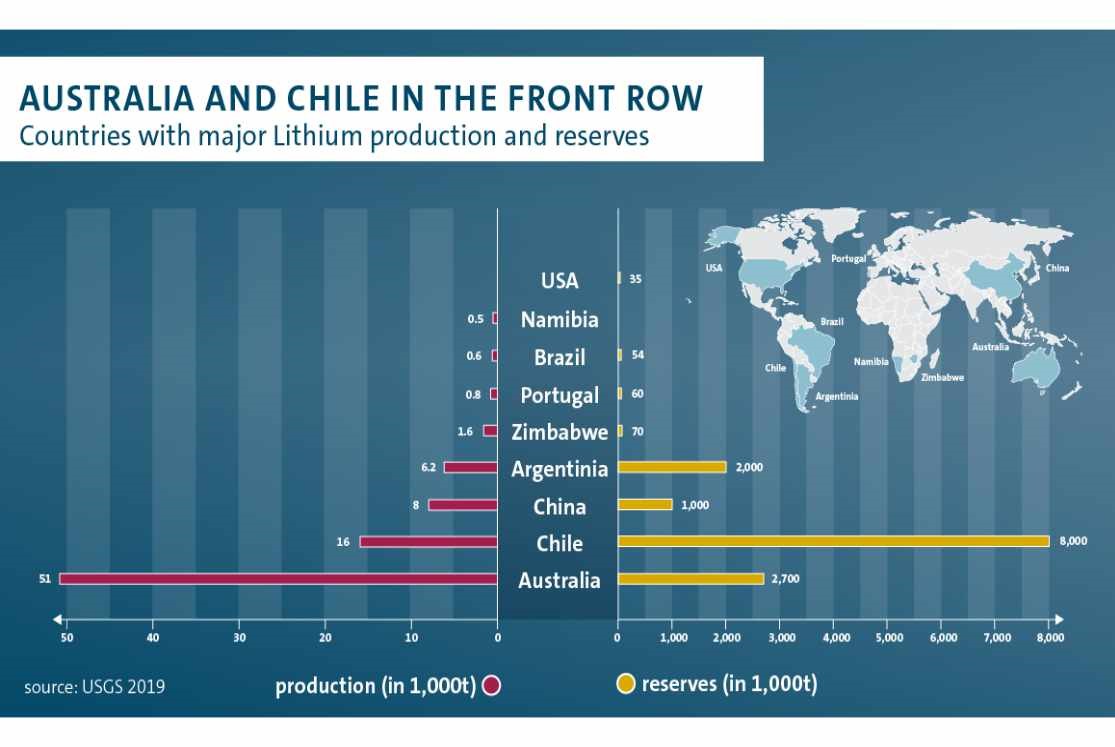
Most of the world’s lithium currently comes from dry ore mining in Australia, but Chile and Argentina have much greater lithium reserves within underground brine deposits.
Brine mining can be a more environmentally sustainable solution than traditional mining
For several reasons, brine mining is usually considered more environmentally sustainable than dry-ore mining:
- Brine mining can be less energy intensive than other forms of mining for the same minerals, as the salty water acts as a natural solvent and makes it easier to extract and process compounds compared to processes that involve the extraction of ores from hard rock.
- Brine mining does not require the excavation of large amounts of land, which can significantly impact local landscapes and ecosystems. However, extracting large volumes of water from underground deposits does affect the water table and can lead to land subsidence.
- The highly saline environments in which brine is found are inhospitable to most plant and animal life, so brine mining’s impact on biodiversity is minimal.
In addition to the mineral extraction business, many industrial and mining processes use brine mining as part of their wastewater management. Depending on the chemical composition of the brine, brine mining can play a role in both minimum liquid discharge (MLD) and zero liquid discharge (ZLD) wastewater treatment strategies to comply with relevant legislation. Instead of disposing brine effluents into bodies of water or sewer systems, injecting them into deep wells, or letting them evaporate in ponds, wastewater brines are treated in a variety of ways to concentrate it to decrease its volume and disposal costs and derive valuable chemicals – in addition to fresh water.
In a circular economy context, brine mining is more than a potential add-on to MLD and ZLD strategies. It can also provide financial and compliance incentives for wastewater treatment, and, in some cases, even pay for itself by transforming, at least in part, harmful waste products into useful byproducts.
The same forces that drive brine mining growth in general will drive its growth in connection with SWRO
With its estimated volume of approximately 1.3 billion cubic kilometers, the world’s most ubiquitous brine is, of course, seawater.
Broadly speaking, interest in brine mining in conjunction with SWRO is motivated by the same two factors discussed above: for-profit extraction of minerals and compounds with commercial value and managing wastewater to minimize environmental impact.
Brine mining seawater for profit
- Demand for the minerals and compounds found in seawater continues to grow with increased industrial and agricultural output.
- Six ions comprise approximately 99% of all sea salts by weight: chloride (Cl−), sodium (Na+), sulfate (SO24−), magnesium (Mg2+), calcium (Ca2+), and potassium (K+). Sodium chloride is the most prevalent seawater chemical with ionic bonds, but seawater also contains many other dissolved salts with commercial value, albeit in smaller volumes.
- Because of its prevalence, sodium chloride is the most valuable chemical that can be extracted from seawater. The primary industrial use of sodium chloride is to produce chlorine and caustic soda, which are used to manufacture a variety of products, including plastics, textiles, and paper. But sodium chloride is also used in the chemical industry to produce sodium hydroxide and hydrochloric acid, as well as in the oil and gas industry to extract natural gas and remove impurities from oil. In addition to its industrial uses, sodium chloride is, of course, also a common ingredient in food. However, only about 8% of worldwide sodium chloride production is used in the food industry, either as table salt or as an ingredient in processed foods.

-
Magnesium is another valuable chemical that can be extracted from seawater. It is used in the production of magnesium metal and in various other applications in the pharmaceutical and agricultural industries.
-
Potassium is used in the production of fertilizers, as well as in a variety of other applications, including in the food industry and in medicine.
-
Bromine has a wide range of uses in various industries, including water treatment, flame retardants, chemical production, and the food industry. Its versatility and effectiveness make it an important and valuable chemical element.
Mitigating SWRO’s environmental impact through brine mining
RO brine, also known as reject or concentrate, is a waste product of SWRO. Indeed, its disposal back into the seas from which the seawater was extracted is a controversial issue in some quarters, but not everywhere. Some argue that it has no practical impact on the environment; others maintain that it has extremely negative effects on marine life. While the debate is ongoing and beyond the scope of this blog, it is clear that better science and more studies are needed to fully understand the impacts of brine discharge.
At any rate, there is no doubt that brine mining has the potential to reduce the amount and concentration of SWRO brine that is discharged into the ocean as part of a ZLD or MLD strategy.
Which brine concentration technology is the right one?
Brine mining depends on brine concentration technologies, essentially the same used in MLD and ZLD, to reduce brine volume prior to the evaporation and crystallization processes that yield dry solids. Solar evaporation is typically used to the extent possible, but membrane systems are always necessary.
In addition to the reverse osmosis (RO) common to SWRO, several other membrane technologies are emerging to concentrate brine. Each of these technologies has its advantages and disadvantages relative to what brine elements can be concentrated, optimal pressure ranges, and membrane scaling effects. These brine-concentrating technologies are either pressure- or electrically driven:
Pressure-driven:
- Osmotically Assisted Reverse Osmosis (OARO)
- Nanofiltration (NF)
- Ultra-high pressure Reverse Osmosis (UHPRO)
Electrically-driven:
- Monovalent Selective Electrodialysis (MED)
- Electro Metathesis (EDM)
- Bipolar Electrodialysis (BPED)
As yet, there is no general consensus on which of these technologies will eventually dominate brine mining in connection with SWRO. All are considered to be ripe for further technical optimization that will impact brine mining and could have broader implications for the desalination industry.
In its massive Jubail 2 project, SWCC will use a dual brine concentrator that deploys nanofiltration prior to the SWRO process to first remove most of the seawater’s calcium and magnesium, and then further concentrate the highly saline RO reject. It will then crystallize the 99.6% pure sodium chloride and bromine for use in Saudi industries.
Will brine mining mean changes for SWRO operators?
Brine mining’s disruption of the SWRO industry has begun, but no one yet knows where this process will take us.
Clearly, the advantages of scale will matter, and the impact of change is likely to begin with very large SWRO producers. For example, the business case for extracting and selling two million dry tons of high-quality salt per year, as SWCC’s Jubail 2 plant intends to do, is made possible only by combining the brine mining investment with a greenfield, one million m3/day SWRO plant. At present, this is not something for small and medium SWRO producers. But this, too, could change.
Another likely impact of brine mining on SWRO will concern technological innovation. As brine mining grows in connection with SWRO, wastewater treatment, and mineral mining as such, the market for brine concentration technology will also grow. Membrane producers will compete to develop better anti-scaling solutions and ways to do more with less pressure. EPC contractors will vie for projects that depend on new technologies. All this innovation will not only shape the emerging SWRO-brine-mining industry – it will also usher in innovation that could also disrupt “traditional” SWRO, too.
Indeed, the combination of brine mining and SWRO might cause some to ask, “What business are we in?” Will mining companies begin to ramp up their activities in brine mining and sell fresh water as a byproduct? Will SWRO companies become mining companies? Will new companies be born with a foot in both industries?
Another open question regards cost and affordability. What will brine mining mean for the price of SWRO water? Will new business models make SWRO even more affordable for coastal communities in developing countries that are hardest hit by climate change and water scarcity?
And what will brine mining mean for permitting and compliance? For example, will regulators require more MLD and ZLD solutions to greenlight SWRO projects?
One thing is certain: The combination of brine mining and SWRO will look very different in 10 years than it does now!
Related blogs
Read more about our solutions for desalination
-
if (isSmallPicture) {


 High-pressure pumps for SWRO applications
High-pressure pumps for SWRO applicationsThe range of high-pressure APP pumps is optimized for both landbased, off-shore and marine sea water reverse osmosis applications. Available with or without motor.
-
if (isSmallPicture) {


 Energy recovery device for medium to large SWRO applications
Energy recovery device for medium to large SWRO applicationsThe first active ERD for medium and large plants integrates highly effective isobaric pressure exchangers with a low-voltage motor to eliminate the risk of rotor overspin, reduce mixing and biofouling, and facilitate smarter automation. Covering train sizes from 1,500 m3/day and above.
-
if (isSmallPicture) {


 iSave® energy recovery devices for high-pressure membrane applications
iSave® energy recovery devices for high-pressure membrane applicationsWith a 3-in-1 design that integrates highly effective isobaric pressure exchangers with positive displacement booster pumps and electrical motors, iSave® ERDs deliver big energy savings in small spaces. Covering train sizes from 200-3,000 m3/day
-
if (isSmallPicture) {


 VLT® AQUA Drive FC 202
VLT® AQUA Drive FC 202VLT® AQUA Drive FC 202 controls all types of pumps and comes equipped with a cascade controller.
-
if (isSmallPicture) {


 DST P40I titanium pressure transmitter for use in corrosive environments and with aggressive media
DST P40I titanium pressure transmitter for use in corrosive environments and with aggressive mediaFor use in corrosive environments and with aggressive media, Danfoss offers the robust DST P40I pressure transmitter made of Titanium and with ceramic pressure sensing element. DST P40I is optimized for use in applications such as desalination systems, seawater cooling, and chemical processing.























